 https://hemaxis.com/wp-content/uploads/2019/07/logo-lyonbiopole-en.png
216
288
hemaxisdbs
http://hemaxis.com/wp-content/uploads/2016/08/hemaxis-logo-x1.png
hemaxisdbs2019-07-15 11:23:172020-02-10 15:30:08Joining Lyonbiopôle
https://hemaxis.com/wp-content/uploads/2019/07/logo-lyonbiopole-en.png
216
288
hemaxisdbs
http://hemaxis.com/wp-content/uploads/2016/08/hemaxis-logo-x1.png
hemaxisdbs2019-07-15 11:23:172020-02-10 15:30:08Joining Lyonbiopôle https://hemaxis.com/wp-content/uploads/2019/07/logo-lyonbiopole-en.png
216
288
hemaxisdbs
http://hemaxis.com/wp-content/uploads/2016/08/hemaxis-logo-x1.png
hemaxisdbs2019-07-15 11:23:172020-02-10 15:30:08Joining Lyonbiopôle
https://hemaxis.com/wp-content/uploads/2019/07/logo-lyonbiopole-en.png
216
288
hemaxisdbs
http://hemaxis.com/wp-content/uploads/2016/08/hemaxis-logo-x1.png
hemaxisdbs2019-07-15 11:23:172020-02-10 15:30:08Joining Lyonbiopôle
HemaXis™ DB10 samples professional cyclists in drug monitoring program
DBS System SA today announced its collaboration with the Union…

DBS System’s HemaXis™ DB 10 blood collection device is now CE/IVD marked
DBS System SA announced today that its blood collection device,…

Efficient Alcohol Consumption Monitoring
PEth testing is quickly becoming the preferred choice for alcohol…

HemaXis DB launched in the U.S. with FDA listing
DBS System has launched their HemaXis DB micro blood collection…
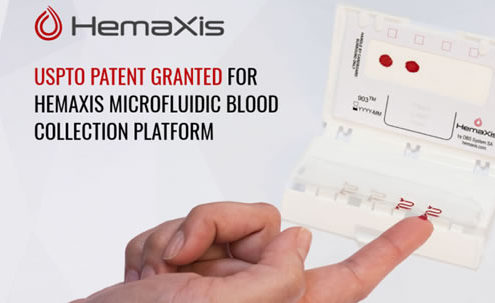
USPTO Patent Granted For HemaXis Microfluidic Blood Collection Platform
We are proud to announce the USPTO patent covering our 1st Generation…
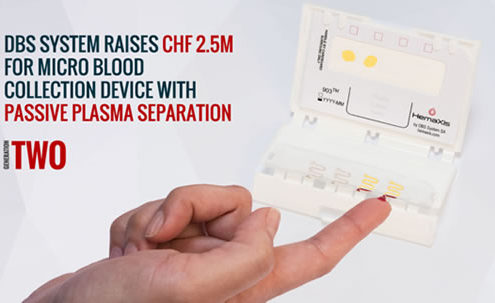
DBS System raises CHF 2.5M to produce revolutionary micro blood collection device capable of true passive plasma separation
DBS System announced it closed its Series A at CHF 2.5M which…

DBS System recognized as a Top 50 Startup by Bilan
The DBS System team is proud to announce that Bilan selected…

Nest investira une part de son capital dans des start-up
La fondation collective entame un partenariat inédit avec la…
DISCLAIMER
HemaXis DB is a FDA Class 1 medical device. HemaXis DX is in development and for research purposes only. Use of HemaXis devices in Laboratory Developed Tests (LDTs) requires further processing including the establishment of performance characteristics and successful validation by the laboratory in a manner consistent with local regulatory requirements.
By using this website you are agreeing to our Terms and Conditions.
CONTACT
DBS System SA
Rte des Avouillons 6
CH-1196 Gland
Switzerland
Tel: +41 (0) 78 624 70 15
Email: info@dbs-system.ch
LEGAL DOCUMENTS
Read Our Privacy Policy
Read Our General Terms and Conditions of Sale
Read Our Shipping Information
